-
Change Request
-
Resolution: Persuasive with Modification
-
Medium
-
US Da Vinci CDex (FHIR)
-
1.1.0-ballot [deprecated]
-
Patient Care
-
STU
-
Background
-
-
Eric Haas/Bob Dieterle: 8-0-3
-
Enhancement
-
Compatible, substantive
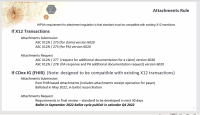
It is unclear from this guide, and the DTR and PAS guide how CDex is used. It would be helpful in either of the guides, if not all, to describe the flow when additional data is necessary how CDEx conveys that the request is for purposes of an existing prior-auth or claim (one or more), and why the technique used to identify the additional data and submission is different (e.g., use of Questionnaire/QuestionnaireResponse in DTR and submission using Claim).
- is duplicated by
-
FHIR-37257 overlap with DV Burden Reduction guides?
-
- Duplicate
-
-
FHIR-36181 Data Sets as "attachments"
-
- Duplicate
-
- is voted on by
-
BALLOT-35127 Negative - Hans Buitendijk : 2022-May-FHIR IG CDex R1 STU
- Closed